Controlled Atmospheric Brazing (CAB)
The aluminum radiators as inexpensive heat exchangers for such an AiO compact water cooling system, if they are to really last, are expediently manufactured (as also in automobile construction) by means of CAB (controlled atmospheric brazing). For this, together with the special process, a non-corrosive flux is used, which is based only on potassium aluminum fluoride. But how does the whole thing actually work?
In the molten state, the special flux (in this case, for example, NOCOLOK© by Solvay Fluor) then transforms into a so-called “ionic liquid”, which in turn attacks and partially removes the interfering oxide layer of the aluminum alloy. However, this requires a special protective atmosphere of nitrogen (< 200 ppm oxygen). But what makes this special flux so special that it does not exert a disturbing influence later on? It is the special mode of action!
Anyone who has been reasonably attentive in chemistry will now also understand that it is harmful if the flux used merely “reduces” the oxide (written in simplified terms) in a “redox reaction”, i.e. a simple exchange of electrons takes place. This cannot be completely stopped later, but only reduced or partially inhibited. The appropriate flux, on the other hand, “dissolves” the oxide completely. This can be compared well with common salt, which dissolves in water.
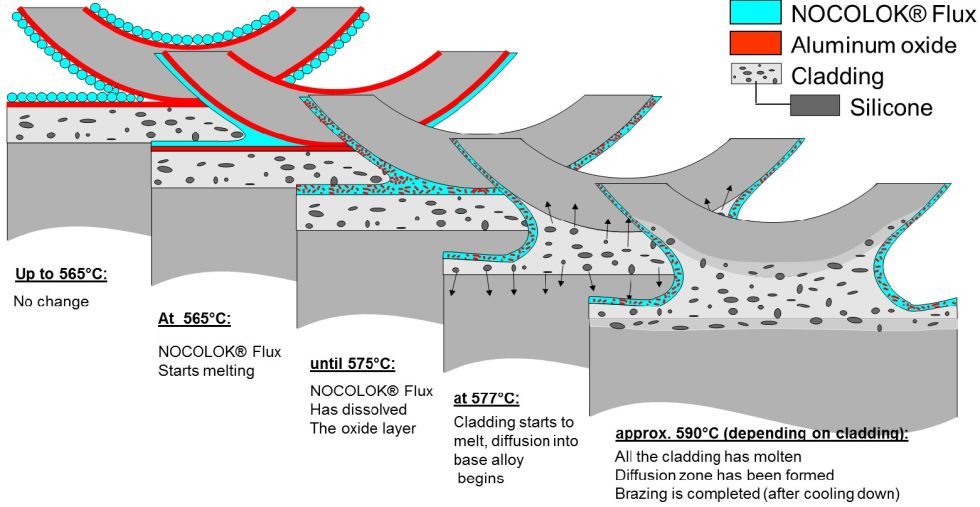
The following figure shows how the applied layer of flux behaves in detail and during the ongoing soldering process at just under 600 °C. The flux is dissolved in water:
The 7 steps, as published by Solvay, show how the individual processes are structured. This process has been used successfully for decades, but also requires somewhat more effort than brazing with corrosive fluxes.
7_Schritte



































283 Antworten
Kommentar
Lade neue Kommentare
Urgestein
Mitglied
Urgestein
Urgestein
Mitglied
1
Mitglied
Urgestein
Mitglied
Urgestein
1
Veteran
Urgestein
Urgestein
Mitglied
Neuling
Urgestein
Urgestein
Neuling
Alle Kommentare lesen unter igor´sLAB Community →