Today we want to find out what is really in the two extremely expensive pastes and what philosophy is behind the respective mixture. And to spoil it a bit in advance: The biggest difference here is really only the color. Chic baby blue in the tube against a nice baby pink from the can and two prices reminiscent of certain products with the gold edge. But it’s like with certain high-speed tires in real life: They have their justification if the intended use fits. For everything else, it’s more wishful thinking and, of course, a bit of a marketing lesson. Even from my side, there is almost honest admiration, because you didn’t think of it yourself.
This time, the two pastes were de facto paid for by the donation box and I hope that I will not be accused of pure waste or misappropriation. But in the end, curiosity was too great to dissect the two precious substances thoroughly. But before we get started with the analysis, I want to get rid of a few little basics that are important to know when evaluating the two pastes.
Thermally conductive particles as a basis
Each paste relies on specific particles of a chemical compound with the lowest possible thermal resistance. There are many options here, up to and including diamond powder. However, they are also particles whose grain size plays a very important role in subsequent performance. On the one hand, there is the shape, which can be platelet-shaped, crystal-shaped or rather round. On the other hand, of course, the grain size also plays a very important role. And that’s where theory and practice already clash. Coarser grits offer lower thermal resistance, at least on paper, but also leave larger gaps between the particles and even the surface. This, in turn, also negatively affects the actual thermal conductivity of the entire mixture.
However, higher grit sizes usually make the coatings a bit too thick. Too fine grits, on the other hand, produce thinner layers under great pressure, but with a bit of bad luck make pastes too solid. Liquid, rather slurpy or a bit more viscous? With the grain size, you can control the consistency quite well in advance. It is clever to mix different grit sizes, i.e. anything between 1 and 2 µm and something larger up to approx. 5 µm. Then you have something from both worlds.
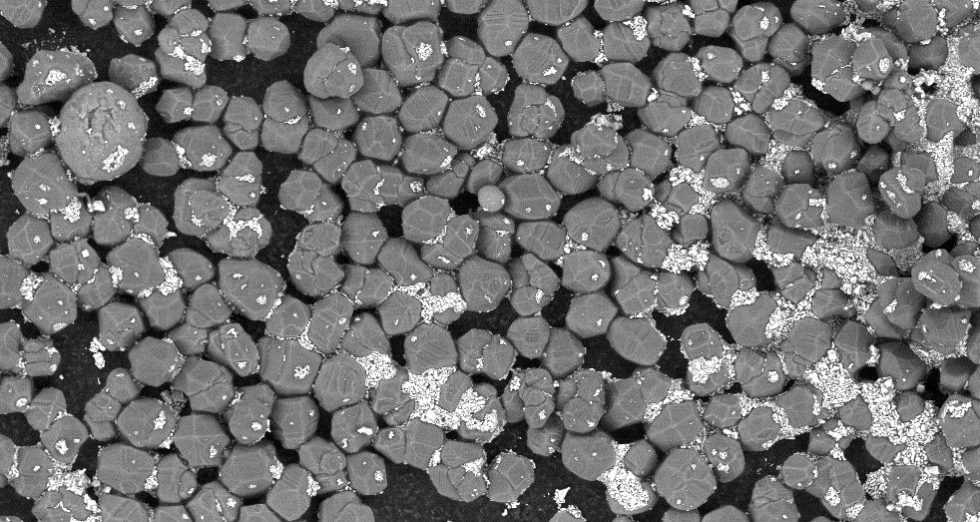
Fillers
And how do you get the spaces between a corundum, for example, filled appropriately? For everything below 1 µm, a filler is used that is still as thermally conductive as possible and is much cheaper, often in the form of platelets. Especially the very fine grits of corundum, diamond and boron nitride are really expensive. The stuff has to be ground in a complex process, and that costs money. Therefore, many set the cut at around 1 to 1.5 µm and fill up with additives. The cheapest are things like zinc oxide, which are also used on their own as a base for cheaper pastes.
Binder and mixture
To ensure that the paste does not reach the customer as dust, various binders are used, which on the one hand guarantee the final consistency of the paste and on the other hand represent one of the most important secrets when it comes to long-term durability (“reliability”) and performance in certain temperature windows. The pastes tested today can also be used for sub-zero applications, which of course poses different challenges for the binder than for a “normal” consumer paste.
The silicone oils (linear polysiloxanes) that are almost always used are, from a purely chemical point of view, a middle ground between inorganic and organic chemistry. They have an inorganic framework (as in rocks and minerals) and contain certain organic residues. This positions them between silicates and plastics. The resulting properties are versatile and, above all, easily controllable. Unfortunately, these silicone oils are difficult to analyze with an EDX. Even if you can use a cryogenic table, you only get the chemical elements, but no information about the molecules themselves and their degree of crosslinking. If one of the readers would be able to: gladly times a feedback by PN in the forum, by Mail or also telephone (stands everything in the imprint).
Very important for the quality and shelf life of a paste as well as the congruent consistency of all batches is “conching”, i.e. the permanent and complete mixing of all ingredients until the ideal distribution of all ingredients is approximately achieved and maintained. The mass distribution will certainly never be perfect, but almost. But you know this from cocoa: if you don’t stir and shake constantly, the suspended solids sink back to the bottom over time and the paste dissolves. The only thing that helps is constant stirring and agitation. If you don’t do this or do it inadequately, you will have a different result for each batch and the pastes will no longer be the same. Especially with cheaper fillers there is really danger ahead, because you can make even the best paste almost useless by such carelessness.


































42 Antworten
Kommentar
Lade neue Kommentare
Urgestein
Urgestein
1
Veteran
Veteran
Veteran
Veteran
Veteran
Veteran
Urgestein
Urgestein
Urgestein
Urgestein
1
Veteran
Urgestein
Mitglied
Veteran
Urgestein
Alle Kommentare lesen unter igor´sLAB Community →